Abstract
Introduction: Venous thromboembolism (VTE) is a cause of significant morbidity and mortality in cancer patients and its prevalence in this population is increasing. The risk of VTE varies with different factors and the Khorana risk model, a clinical VTE risk assessment algorithm, has been developed to predict the risk of VTE in patients with cancer. Information regarding the economic burden associated with the risk of VTE is limited. The current study evaluates healthcare costs associated with different risks of VTE based on Khorana risk scores (KRS) among patients newly diagnosed with cancer.
Methods: The Optum©'s Clinformatics® Data Mart database (01/2012 - 09/2017) was used to select patients ≥18 years with ≥1 hospitalizations or 2 outpatient medical claims with a cancer diagnosis (index date) who initiated chemotherapy or radiation therapy within 45 days of the index date. Patients were also required to have ≥6 months of eligibility prior to the index date (i.e., baseline period), no evidence of a VTE during the baseline period, no anticoagulant therapy used during the baseline period or up until a VTE event, and no evidence of major surgery following the index date. Patient also had to have ≥1 laboratory result for hemoglobin, leukocyte, and platelet counts within 28 days before initiating their cancer treatment. The KRS (calculated using the index cancer site, body mass index, and laboratory results prior to treatment [i.e., platelet, leukocyte, and hemoglobin counts]) was used to classify patients in the following cohorts based on KRS: 0, 1, 2, and ≥3. Patients were observed from the index date up to 12 months post index, end of data availability, death, or end of insurance coverage, whichever occurred first. All-cause and VTE-related healthcare costs (i.e., total healthcare, hospitalization, emergency room visit, and outpatient visit costs) were assessed and reported per-patient-per-month (PPPM) in 2018 USD. VTE-related costs were defined based on claims with a primary or secondary diagnosis of VTE and also included anticoagulant therapy costs. Unadjusted and adjusted cost differences (adjusting for age, sex, year and month of index date, insurance type, Quan-Charlson comorbidity index [CCI] score, Elixhauser comorbidities with a proportion ≥5%, type of cancer at index, and healthcare utilization and costs) were calculated.
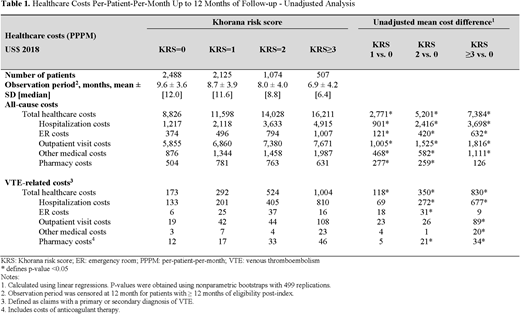
Results: A total of 6,194 patients (KRS=0: 2,488; KS=1: 2,125; KRS=2: 1,074; KRS≥3: 507) were included in this study. The mean age was 68 years, 48% to 52% of patients were female, and the mean CCI ranged from 1.1 to 1.4. The mean follow-up period ranged from 6.9 months for the KRS≥3 cohort to 9.6 months for the KRS=0 cohort. All-cause total healthcare costs PPPM were $8,826 (KRS=0), $11,598 (KRS=1), $14,028 (KRS=2), and $16,211 (KRS≥3) (see Table 1). All-cause hospitalization costs and outpatient visit costs PPPM also increased with VTE risk. Mean unadjusted all-cause total healthcare cost differences were $2,771 for the KRS= 1 vs 0 group; $5,201 for the KRS=2 vs 0 group; and $7,384 for the KRS≥3 vs 0 group. All-cause hospitalization cost differences were $901 for the KRS=1 vs 0 group; $2,416 for the KRS=2 vs 0 group; and $3,698 for the KRS≥3 vs 0 group. Likewise, all-cause outpatient visit cost differences PPPM were $1,005 KRS=1 vs 0 group; $1,525 for the KRS=2 vs 0 group; $1,816 for the KRS≥3 vs 0 group. Similar patterns were observed for VTE-related healthcare cost differences between cohorts. Adjusted analyses yielded similar cost differences between cohorts.
Conclusions: Patients newly diagnosed with cancer, who are at a higher risk of a VTE, experienced significantly higher all-cause and VTE-related healthcare costs compared to patients with a lower risk of VTE. VTE-related costs represented 2% to 6.2% of the total costs for KRS=0 to KRS≥3, and were mainly driven by VTE-related hospitalization costs. Part of these costs and consequences of VTE could potentially be reduced in the higher risk subgroups with outpatient prophylaxis
Kuderer:Myriad Genetics: Consultancy; Pfizer: Consultancy; Mylan: Consultancy, Other: Travel, Accommodations, Expenses; Celldex: Consultancy; Halozyme: Consultancy; Coherus Biosciences: Consultancy, Other: Travel, Accommodations, Expenses; Janssen Scientific Affairs, LLC: Consultancy, Other: Travel, Accommodations, Expenses. Milentijevic:Janssen Scientific Affairs, LLC: Employment, Equity Ownership. Germain:Janssen Scientific Affairs, LLC: Research Funding. Laliberté:Janssen Scientific Affairs, LLC: Research Funding. Le:Janssen Scientific Affairs, LLC: Research Funding. Lefebvre:Janssen Scientific Affairs, LLC: Research Funding. Lyman:Generex Biotechnology: Membership on an entity's Board of Directors or advisory committees; Halozyme; G1 Therapeutics; Coherus Biosciences: Consultancy; Amgen: Other: Research support. Khorana:Bayer: Consultancy; Pfizer: Consultancy; Sanofi: Consultancy; Janssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal